Corrective and Preventive Actions (CAPA) Supplier Corrective Action Request (SCAR)
Automating the Corrective and Preventive Actions (CAPA) Supplier Corrective Action Request (SCAR) with Lyons Quality Audit Tracking System (LQATS) effectively extends the corrective and preventive action (CAPA) compliance process to suppliers, streamlining all tasks related to corrective action process and reduces audit time, improves product quality and safety, and ensures regulatory compliance and improves relationship with suppliers.
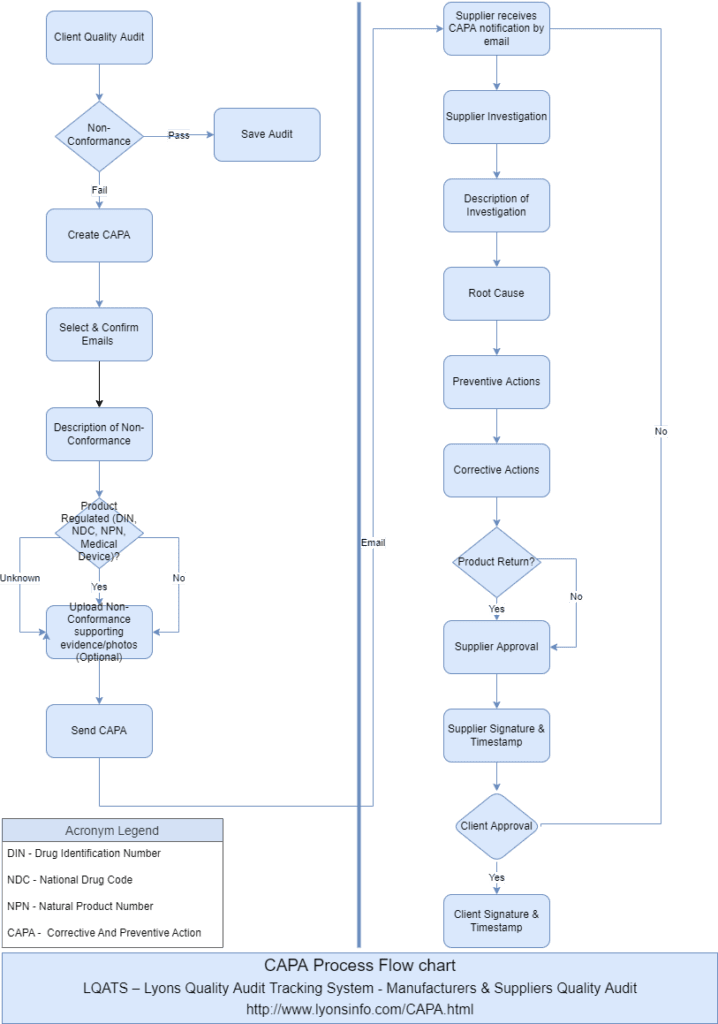
The Lyons Quality Audit Tracking System (LQATS) ensures that suppliers address corrective action requests and other quality events completely while creating a permanent record of activity for each supplier. LQATS utilizes configurable workflows to activate supplier corrective action request procedures, making the process and its results repeatable and measurable by tracking the incidence of corrective actions and response times promptly. By integrating supplier corrective actions with the supplier quality management framework, LQATS enforces higher vendor standards.
Pre-configured forms facilitate the collection and tracking of data throughout the supplier corrective action process.
Corrective and Preventive Actions (CAPA) Home Screen
- The process involves a request phase, supplier collaboration and response, disposition, and Supplier Review Group approval.
- During the request phase, the system captures supplier and product information, issue descriptions, and expected responses.
- A supplier representative or personnel interfacing with the supplier can enter the supplier’s response directly.
- The system captures all key elements of a CAPA process, including containment, root cause analysis, corrective and preventive actions, and verification of completion.
- In the disposition phase, internal personnel assess the sufficiency of responses easily. They may send the supplier corrective action back for clarification or to request additional responses. Internal verification tasks can trigger, and the audit schedule can be reviewed.
Corrective and Preventive Actions CAPA Non-Conformance Detail
- The Supplier Review Group holds final approval responsibility for each supplier corrective action, ensuring appropriate accountability in the quality system.
Supplier Corrective Action Request (SCAR):
Supplier Corrective Action Request (SCAR) A Supplier Corrective Action Request (SCAR) formally requests that a supplier correct a problem and explain exactly how they will do so. This problem may arise from a nonconformity related to the quality of the product. Clients may also ask suppliers to identify the root cause, contain the problem, rectify the issue, and outline further actions to prevent future problems. LQATS creates permanent records around quality events, corrective actions, and supplier deviations, ensuring that the system handles each issue completely while maintaining a record of activity for every supplier.
- Users will have a button “Send CAPA” in the Audit screen when it’s on Edit Mode.
Send CAPA - CAPA form will remain same/standardized for all areas.
- CAPA Non Conformance Detail
Lyons Quality Audit Tracking System LQATS enables clients to maintain compliance by keeping quality systems continuously ready for inspections and audits in collaboration with suppliers. LQATS automates all tasks related to supplier corrective actions, including routing, notifications, follow-ups, escalations, and approvals. This automation simplifies the compliance environment, making it easier to maintain adherence. Central visibility and collaboration, along with automated due date-driven workflows, alerts, and escalations, ensure timely resolution of CAPAs.
A central web and mobile-based repository for all documents related to supplier corrective actions simplifies the search and retrieval process during audits or inspections.
Once the user clicks the “Send CAPA” button, the supplier receives an email with a link.
- CAPA Supplier Email
CAPA Supplier Investigation

Suppliers will see a warning message if their CAPA details are due in three days.
They will receive email reminders for any overdue CAPA details.
The Supplier CAPA Review Group will also receive email notifications for any past due CAPA.
Once a supplier completes the CAPA details, they must sign the form and press the Submit button.
After submission, they cannot update any data.
CAPA Supplier Signature With Timestamp
After the Supplier Review Group completes its review, the electronic signature (eSignature) will be posted and submitted, marking the corrective action as complete.
The supplier will receive a notification email. The system may send the supplier corrective action back for clarification or additional responses.
Each action will be date and time-stamped. Once suppliers submit the CAPA, users can view the PDF file in the existing File Details screen.
CAPA Approval
View Corrective and Preventive Actions (CAPA) Status:
The incidence of corrective action response times ranks among the many metrics for evaluating supplier performance. LQATS allows Cintas to enforce higher vendor standards. The system’s analytics capabilities are vital for supplier CAPA management, providing users with online access to data that trends and filters all important CAPA information collected about suppliers.
Assigned Role Users will be able to see below “View CAPA Status” link the New Menu option.
Storage of completed Corrective and Preventive Actions (CAPA):
CAPA reports is filed in a centralized data store to ensure awareness among quality personnel.
LQATS will give users online access to audit trail that allow them to dynamically view all important CAPA audit trail information.